Claim Value-Added Tax Back
VAT compliance is a critical aspect of conducting global clinical trials successfully. Collaborating with a medical Importer of Record is pivotal in ensuring compliance with VAT regulations and other import-related tax requirements.
We know that clinical trials are costly endeavors, and without meticulously planning and outlining the trial’s entire budget, you can quickly run into a financial crisis before the trial is over. Ensuing VAT compliance and obtaining the maximum tax savings is a specific line item that both sponsors and CROs overlook and end up excluded from the budget.
This is highly unfortunate, as the beauty of the expense is that up to 100% can be reclaimed back if done correctly and essentially save you money. However, clinical trial stakeholders often lose out due to various reasons.
VAT Compliance and Tax Savings
Ensuring VAT compliance and trying to reclaim VAT can be complex, and you can face several challenges, leading to some clinical trial stakeholders not being able to reclaim VAT at all. Here is a breakdown of some of the main reasons:
- Lack of VAT compliance knowledge
- VAT and tax are highly dense subjects with nuances and intricacies that require extensive knowledge. Not everyone within the clinical trial supply chain has this tax knowledge, especially regarding specific rules for research and development activities. Beyond this, each country has its own VAT regulations, which can vary drastically from the next – complicating things even more.
- Time and resources
- Trying to reclaim VAT comes with a lot of paperwork and involves collecting and managing a substantial amount of documentation, invoices, and records. To stay on top of all this would require dedicated personnel familiar with VAT compliance regulations – a resource that sponsors and CROs rarely possess, and it is not feasible for smaller trials.
- Complexity
- It goes without saying that VAT is complex, and the effort it takes to understand all the intricacies of VAT registrations, deadlines, exemptions, rates, and invoicing formats is not only daunting but also time-consuming. Not to mention that each country has to meet its own local regulations.
- Overclaiming or underclaiming VAT
- Uncertainty around which expenses actually qualify for VAT recovery adds another level of complexity for clinical trials. The fear of making a mistake and overclaiming or underclaiming VAT can prevent trials from even attempting to reclaim this cost.
Want a more fundamental overview of VAT reclaim in the clinical trial sphere?
With these reasons in mind, it is understandable why clinical trials lose out on their VAT reclaim possibilities. However, you do not need to break your budget. When you enlist the services of a medical IOR specializing in VAT compliance and tax savings, you secure your bottom line.
The Role of Importers of Record (IOR) in VAT Compliance
An Importer of Record (IOR) is a legal entity responsible for importing goods into a foreign country. In the context of global clinical trials, an IOR plays a crucial role in ensuring VAT compliance by acting as the responsible party for all import-related matters.
With TecEx Medical’s expertise, clinical trial sponsors and CROs overcome the complexities of VAT and customs clearance. This results in minimized risks, delays, and financial burdens. By prioritizing VAT compliance and partnering with us, CROs and sponsors can focus on advancing science and bringing life-saving treatments to patients worldwide.
Want to get started?
Advantages of Using a VAT Compliance Specialist
When conducting global clinical trials, partnering with a specialized medical Importer of Record, such as TecEx Medical, can offer several benefits in terms of VAT compliance and overall import compliance:
- Expertise in VAT Compliance and Import Regulations:
- As a medical Importer of Record, we are well-versed in different countries’ import and VAT regulations. We can accurately determine the applicable VAT rates, exemptions, and documentation requirements. Thus alleviating your burden and minimizing the risk of non-compliance. Through various VAT registrations, we manage the entire process for you.
- Seamless Customs Clearance:
- We handle the customs clearance process efficiently. We ensure that all necessary VAT-related documentation is prepared correctly and submitted to customs authorities. This reduces the chances of shipment delays and unexpected costs.
- Reduced Financial Risks and Financial Transparency:
- Non-compliance with VAT regulations can lead to hefty fines and penalties. By engaging with TecEx Medical, clinical trial sponsors can mitigate financial risks associated with VAT errors.
- Local Knowledge and Relationships:
- We have established networks and relationships with local authorities and suppliers. This local expertise enables smooth navigation of VAT requirements and streamlines the import process.
Ensuring Overall Tax Compliance
Beyond VAT, Importers of Record also contribute to overall tax compliance during global clinical trials. We assist in managing other import taxes, duties, and levies applicable to trial-related materials. Clinical trial sponsors can ensure comprehensive tax compliance by working with an IOR while focusing on their primary research objectives.
After years of navigating the industry, we have seen that clinical trials and pharmaceutical companies often stumble upon two common mistakes holding back their VAT savings:
- Overclaiming VAT
- Underclaiming VAT
Fortunately, we exist, so these challenges don’t.
Have a trial in mind?
TecEx Medical VAT/GST Compliance Solution
Clinical trials are expensive, particularly cross-border trials. Having an efficient tax structure can make your budget go a lot further. This is especially true if you are planning DCTs, as multiple markets will be at play. This means multiple opportunities to reclaim VAT or Goods and Sales Tax (GST). All of which will have significant impacts on your budget.
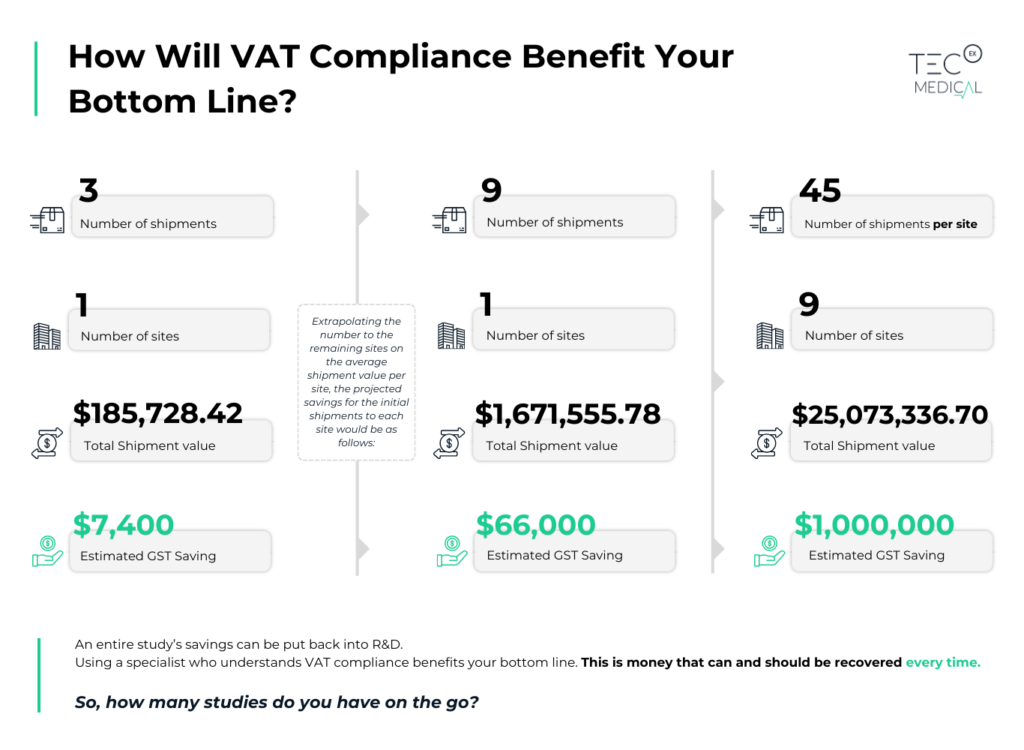
TecEx Medical recognizes the challenges associated with navigating VAT regulations and the significant impact they can have on your clinical trial supply chain. To address these challenges, we provide a unique VAT/GST savings solution available in 33 countries worldwide, including the EU, UK, Australia, and New Zealand.
By involving TecEx Medical from the beginning, we can ensure that the transaction is structured in such a way as to ensure maximization in countries that offer tax exemptions for clinical trial commodities. As the IOR, we apply for the reduced duty, resulting in large savings in countries that do not offer foreign tax reclaim but could still result in large savings for a clinical trial.
With proper planning, negative financial impacts such as irrecoverable VAT may be mitigated. By being involved as the IOR from the start, TecEx Medical will structure the transaction to ensure VAT compliance and that the tax is recoverable.
Ensure VAT Compliance
TecEx Medical offers a one-stop solution. Through our IOR service, we have several value-adds that benefit our clients – from VAT compliance to liability cover. Not only will this reduce the overall cost, but we’ve actually seen occurrences where our clients have ended up net-positive after the VAT reclaim savings have been factored in.
Our customized solution provides compliant importation, VAT handling, and streamlined paperwork management for your clinical trial supplies. So why lose out on what is owed to you? Ensure VAT compliance and recoup those tax savings for your R&D budget.
FAQs
What is overclaiming VAT?
As companies expand their VAT registrations, keeping up with compliance and figuring out what expenses are deductible becomes more challenging. This sometimes leads to including non-deductible expenses in their local returns. This not only results in rejected claims but also opens them up to potential penalties, fines, and audits. Plus, it’s a real drain on resources and time to deal with the fallout.
What is underclaiming VAT?
Managing input VAT processes and staying compliant can be a real drain on resources. Many companies simply don’t have enough time or expertise to maximize their input VAT potential fully.